Gases are all around us, and their behavior is governed by the laws of physics. Ideal gases are often used to simplify calculations, but in reality, most gases behave as real gases, which have different characteristics and behaviors. Understanding the differences between ideal gas vs real gas is crucial in many fields, including chemistry, engineering, and atmospheric science.
What are Ideal Gases?
Definition Of Ideal Gases
The concept of ideal gases is a fascinating and intricate one. Ideal gases are used to simplify calculations related to gases. These gases are composed of numerous minuscule particles that have no volume, are not attracted to one another, and have perfectly elastic collisions. The purpose of ideal gases is to act as a benchmark for real gases, which have more complex behavior due to intermolecular forces, molecular size, and other factors.
Characteristics Of Ideal Gases
Ideal gases have several characteristics that distinguish them from real gases. The most notable is their infinite volume, allowing them to expand and fill any container without exerting any pressure. Additionally, ideal gases have no intermolecular forces, which means that their particles do not interact with each other, granting them the freedom to move independently. Lastly, ideal gases exhibit perfectly elastic collisions, ensuring that no energy is lost when particles collide with each other or with their container’s walls.
Ideal Gas Law (PV = nRT)
The ideal gas law is an essential tool for describing the behavior of ideal gases. It relates the pressure, volume, temperature, and number of particles of a gas, mathematically expressed as PV = nRT. The gas constant, R, is a vital factor in this equation. The ideal gas law is versatile and can predict the behavior of an ideal gas under different conditions, such as changes in pressure, temperature, or volume.
Limitations of ideal gases (only applicable under certain conditions)
Despite its usefulness, the ideal gas law has limitations. Ideal gases are only applicable under specific conditions, such as low pressure and high temperature. When exposed to extreme conditions, real gases deviate from ideal behavior, as factors such as intermolecular forces and molecular size come into play. Thus, other models, such as the van der Waals equation, are used to describe the behavior of real gases under non-ideal conditions.
Read more >> Where Does Glycolysis Occur in The Cell?
What are Real gases
Real gases – those that deviate from the simplicity of ideal gases – exist in a complex and varied realm that transcends the boundaries of our expectations. With characteristics that diverge from those of ideal gases, the behavior of real gases is subject to a multitude of factors and parameters that can bewilder even the most seasoned of scientists.
Definition of Real Gases
The definition of real gases is as enigmatic as the gases themselves. They are gases that possess volume and are subject to intermolecular forces – the mysterious attractive and repulsive forces that govern the interactions between molecules of the gas. However, unlike their ideal counterparts, real gases are subject to collisions that are far from perfectly elastic, meaning that some of the kinetic energy is lost during collisions.
Characteristics of Real Gases
The world of real gases is one in which volume is far from negligible. The volume of gas molecules cannot be ignored in comparison to the volume of the container in which the gas is housed. Consequently, the intermolecular forces that real gases experience can lead to the formation of liquids or solids under certain conditions, particularly when the temperature is low or the pressure is high.
Additionally, the collisions that real gases experience are far from uniform, with the kinetic energy of molecules lost during the process. This characteristic leads to a lack of uniformity in the behavior of the gas as a whole, making it unpredictable and difficult to model.
Deviations from Ideal Gas Behavior
The deviations from ideal gas behavior are one of the hallmarks of real gases. To account for this deviation, scientists have developed the compressibility factor Z, which takes into account the effect of intermolecular forces and the volume of the gas molecules. Alternatively, the van der Waals equation, a modification of the ideal gas law, factors in the volume and intermolecular forces of the gas.
Other Factors Affecting Real Gas Behavior
Other factors, such as temperature and pressure, can also affect the behavior of real gases. For example, the temperature of the gas can impact the average kinetic energy of gas molecules, leading to fluctuations in pressure and volume. Similarly, changes in pressure can have an effect on the intermolecular forces between molecules and their behavior.
In addition, the size of the gas molecules can affect real gas behavior, with larger molecules experiencing different intermolecular forces and a greater number of collisions. The unpredictable and complex nature of these factors renders real gas behavior a perplexing and mystifying realm that defies easy categorization and prediction.
Comparison Between Ideal Gas vs Real Gases
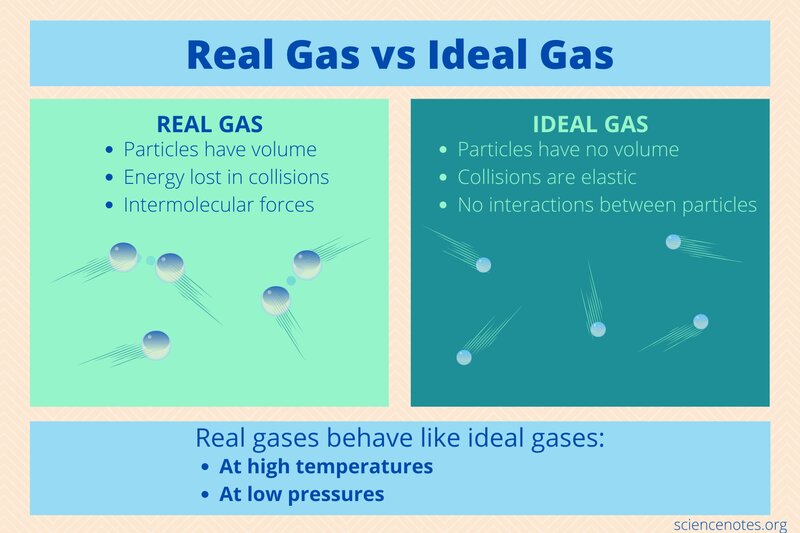
Ideal gases are ideal for theoretical models, but the reality of working with real gases is replete with unique challenges. The differences between ideal and real gases are manifold, including but not limited to:
Volume and Size
Ideal gases are conventionally assumed to have no volume, implying that gas particles do not possess any inherent size. However, this assumption neglects the reality that gas particles actually do have a finite size. As a result, the volume of real gases cannot be disregarded entirely. In fact, at low temperatures and high pressures, the volume of gas particles becomes a critical factor.
Intermolecular Forces
Another striking divergence between ideal and real gases is the existence of intermolecular forces. Ideal gases are devoid of any intermolecular forces that would otherwise attract or repel gas particles. Conversely, real gases exhibit both attractive and repulsive forces between gas particles, which can significantly impact their behavior. The forces between particles can cause them to clump together, leading to a decrease in the number of collisions, and ultimately, a reduction in pressure.
Collisions
The collisions between gas particles are yet another feature that differentiates ideal and real gases. While ideal gases are deemed to undergo perfectly elastic collisions, where no kinetic energy is lost during particle collision, real gases are unable to exhibit the same level of energy preservation. In reality, these collisions are non-elastic, leading to the loss of some kinetic energy as heat. This outcome can bring about alterations in temperature and pressure, leading to an altered gas behavior.
Calculation of Gas Properties Using Ideal Gas Law vs. Real Gas Equations
Given the fundamental differences between the characteristics of ideal and real gases, it becomes patently clear that the ideal gas law may not always be a precise representation of real gas behavior. As a result, it is necessary to turn to real gas equations such as the van der Waals equation, which accounts for particle volume, intermolecular forces, and non-elastic collisions. With these factors factored into the calculations, real gas equations offer more accurate predictions of real gas behavior than the ideal gas law.
Real-World Examples of Ideal and Real Gases
Real and ideal gases abound in everyday life, with helium in a balloon serving as an instance of an ideal gas, primarily due to the negligible volume of the gas particles. Conversely, the air in a scuba diving tank behaves more like a real gas, given the low temperatures and high pressures that cause gas particles’ volume to become more significant. Moreover, refrigeration systems rely on the characteristics of real gases, primarily because they require a gas that can be compressed and expanded without losing energy.
Applications of Real Gas vs Ideal Gas Laws
Use of Ideal Gas Law in Engineering and Scientific Applications
It is well-established that ideal gas law finds application in a plethora of engineering and scientific fields. An excellent example of this is the use of the ideal gas law in designing combustion engines, gas turbines, and industrial processes that involve gases. The ideal gas law permits engineers to compute the required volume, pressure, temperature, and amount of gas necessary for any given process or application.
Furthermore, the design of Heating, Ventilation, and Air Conditioning (HVAC) systems relies heavily on the use of ideal gas law. It facilitates the computation of the necessary refrigerant amount that would ensure a space is appropriately heated or cooled.
Real Gas Behavior in Chemical Reactions and Gas Storage
The intricate behavior of real gases must be taken into consideration to ensure accuracy in predicting and optimizing conditions for chemical reactions. Chemists must be cognizant of the intermolecular forces between gas molecules since they can impact the rate and direction of chemical reactions. Therefore, an understanding of the complex nature of real gas behavior is critical for optimizing and designing chemical reactions.
Also, in gas storage, the compressibility factor is used to accurately calculate the volume of gas stored in tanks and pipelines, considering the deviation of real gases from ideal gases. This is a prerequisite for ensuring the proper functioning of gas storage and transportation systems and for safety reasons.
Impact of Real Gas Behavior on Climate and Atmospheric Science
Real gas behavior plays a pivotal role in understanding climate and weather patterns in atmospheric science. For instance, the greenhouse effect, which accounts for the warming of the Earth’s surface, is primarily caused by the behavior of real gases like water vapor, carbon dioxide, and methane.
An understanding of real gas behavior is essential in comprehending the properties of the Earth’s atmosphere, such as its composition and pressure. Scientists utilize real gas equations to model the behavior of gases in the atmosphere, which is a prerequisite for predicting future climate change and developing mitigation strategies.
Conclusion
In conclusion, while ideal gases are useful for simplifying calculations, most gases in the real world behave as real gases, which have different characteristics and behaviors. By understanding the difference between ideal gas and real gas, we can make more accurate calculations and predictions in many fields, from engineering and chemistry to atmospheric science. The study of gas laws is ongoing, and as we continue to refine our understanding of gas behavior, we will be able to apply this knowledge to an even wider range of real-world applications.

PCCN vs CCRN: Which Certification Should I Take?
In this discussion, we will examine the fundamental distinctions between PCCN vs CCRN certifications, allowing you to make an informed and right decision about which certification is best for your nursing career progression.
June 20, 2023

Is PCCN Worth It? A Comprehensive 2024 Study Guide
In this article, we will provide all the enrollment criteria, how to apply, whether is PCCN worth it for you to obtain, and how to get a high mark.
June 20, 2023

PCCN Requirements - How to Become a Progressive Care Certified Nurse?
To become a progressive care nurse, you must first obtain the PCCN certification. This post will help you understand PCCN certification, PCCN requirements, and efficient approaches to obtaining this certification.
June 20, 2023
