Aiming to succeed on the HESI A2 Chemistry exam and seeking assistance with your exam preparation? This blog post on the Chemistry Study Guide is for you! We will talk about what a HESI A2 Chemistry Test entails. Afterwards, we’ll give you some study advice to make sure you ace your test!
After learning everything in this study guide, test your understanding with our free HESI A2 practice test 2024 to solidify your knowledge!

What’s on the HESI A2 Chemistry Test?
If you are thinking of applying to a nursing school, the HESI A2 Chemistry test may be coming up in the near future. Many nursing programs require all applicants to take the HESI A2 exam as part of the admissions process. The HESI A2 consists of seven major components, and schools can select which of the seven they want applicants to take. Many schools do not require applicants to take Chemistry, but many do. If you are unsure whether the school to which you are applying requires it, you should check with them.
There are 30 multiple-choice questions about chemistry though only 25 of those questions count toward your score. In most cases, you will have 25 minutes to complete the HESI Chemistry test, but schools can establish their own restrictions, so you should verify the actual allotted time with your school before taking the exam.
HESI A2 Chemistry Study Guide
Units of Measurement
While most measurements in nursing use the metric system, you may need to be able to approximate the US and/or Imperial measurements as a comparison.
Volume
Volume is usually measured in milliliters (ml) or cubic centimeters (cm3).
- 1 ml = 0.001 liter
- 1 ml = 1 cm3
For example: 6L = 6000 ml = 6000 cm3
For larger quantities, US gallons are used in the US, and Imperial gallons are used in the UK. Some helpful conversions are:
- 1 Imperial gallon = ~ 1.2 US gallons
- 1 US gallon = ~ 3.79 liters or 231 cubic inches
- 1 Imperial gallon = ~ 4.55 liters or ~ 277.42 cubic inches
- 1 liter = 2.11 US pints
Mass and Length
Mass is commonly measured in grams (g) or kilograms (kg), where 1 kg = 1000 g.
- 1 kg = 2.2 pounds
- 1 pound = 16 ounces
- 1 stone = 14 pounds
Length is usually measured in meters (m), centimeters (cm), or millimeters (mm).
- 1 inch = 2.54 cm
- 1 foot = 30.5 cm
- 1 yard = 91.44 cm
- 1 km = 0.621 miles
States of Matter
There are four fundamental states of matter – solid, liquid, gas, and plasma. Solids are characterized by closely packed particles, held together by strong intermolecular forces to form a definite shape. Heating a solid to a temperature above its melting point transforms it into a liquid. In liquids, the intermolecular forces are weaker, meaning the particles have more freedom of movement.
By heating a liquid to a temperature higher than its boiling point, it can be turned into a gas. In a gas, the particles have enough kinetic energy to pass through the intermolecular forces and can freely move. Heating a gas to high temperatures can produce plasma. This leads to the electrons in an atom separating from the nuclei and plasma can be thought of as a cloud of free electrons and positively charged ions.
Atoms
Elements are made of atoms, and atoms are made of three types of particles – negatively charged electrons, positively charged protons, and neutral neutrons.
The protons and neutrons make up the nucleus of the atom. The nucleus has a very small diameter compared to the overall size of the atom, but it is where most of the mass is concentrated. The electrons orbit the nucleus in shells and most of the volume of the atom is taken up by the free space between the nucleus and electrons.
An element’s nuclear symbol tells you how many electrons, protons, and neutrons make up each atom.
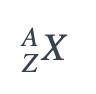
where,
X is the element symbol.
A is the mass number, which tells you the total number of protons and neutrons in the nucleus.
Z is the atomic number, which is the number of protons in the nucleus.
The number of protons is equal to the number of electrons for neutral atoms. The number of neutrons is A – Z. A neutral atom of lithium represented by the nuclear symbol 73Li has three protons, three electrons, and four neutrons, which is the example of the number of protons.
Ions have unequal numbers of protons and electrons. Positive ions have more protons than electrons and negative ions have more electrons than protons.
All atoms of the same element have the same number of protons. Isotopes are atoms of the same element but with different numbers of neutrons. For example, 126C and 136C are both isotopes of carbon.
Acids and Bases
Using the pH scale, we can measure the acidity or basicity of a chemical or solution. The pH scale ranges from 0 to 14 and a pH of 7 is neutral. A solution with a pH lower than 7 is classed as acidic and a solution with a pH higher than 7 is classed as basic.
Hydrochloric acid (HCl), which is part of the acid that is found in the stomach, is a very strong acid with a pH of 1. Water is neutral, with a pH of 7. Sodium bicarbonate (NaHCO3) has a pH of 9, making it a weak alkali. Sodium hydroxide (NaOH) is a very strong alkali and when concentrated can have a pH of 14.
The pH scale is logarithmic with a base of 10. This means that each unit difference corresponds to a change of a factor of 10. pH 3 is 10 times more acidic than pH 4 and 1000 times more acidic than pH 6 is the example for the pH scale mentioned in the following.
Chemical Equations
Balanced equations should have the same number of each type of atom on both sides. You have to alter the number of molecules of one or more compounds on either side of the equation to balance equations. Balance the equation:
C2H6+O2 => CO2+2H2O
This is the first step is to work out the number of atoms on each side:
Left Right
C = 2 C = 1
H = 6 H = 4
O = 2 O = 3
The right side needs 1 more ion of C and 2 more ions of H, so we can add 1 more CO2 compound and 1 more H2O compound and the equation becomes:
C2H6+O2 => 2CO2+3H2O
Left Right
C = 2 C = 2
H = 6 H = 6
O = 2 O = 7
Because we have also added more atoms of O to the right, we now need five more atoms of O on the left to make 7 atoms in total. Add another 2.5 atoms of O2 to the left and the equation is balanced:
C2H6+3.5O2 => 2CO2+3H2O
Left Right
C = 2 C = 2
H = 6 H = 6
O = 7 O = 7
But chemical equations typically do not consist of fractional coefficients, so let’s multiply the entire equation by 2 to eliminate the fraction, 3.5:
2(C2H6)+7(O2) => 4(CO2)+6(H2O)
Chemical Reactions
Particles in liquids and gases are constantly moving and colliding with each other. Under the right conditions, these particles can react. To start a reaction, the particles need to have a minimum amount of kinetic energy, known as the activation energy. This is the amount of energy required to break the bonds within each particle.
The rate of a reaction increases as temperature increases because the particles will have more kinetic energy. This means that they will be moving faster and colliding more often and be more likely to have enough energy to break the activation barrier. Increasing concentration also develops the rate of reaction as this will raise the number of particle collisions.
Catalysts can also be added to raise the reaction rate. These work by lowering the activation energy. In this instance, platinum plays a role of a catalyst in the reaction that makes nitric acid from ammonia. Don’t forget that platinum does not appear in the equation. Because during a chemical reaction a catalyst is not consumed or changed.
NH3+O2 => HNO3
Oxidation and Reduction Reactions
Oxidation is the loss of electrons. Reduction is a gain of electrons. In redox (oxidation and reduction) reactions, oxidation and reduction occur simultaneously.
In the reaction:
2Na+Cl2 => 2Na+Cl
Sodium is oxidized as it loses an electron and chlorine is reduced because it gains an electron – sodium is the electron donor and chlorine is the electron acceptor.
Read more >> The Comprehensive HESI Biology Study Guide
Common mistakes students make while studying for this exam
- Using the wrong book to study from
- Not understanding that this is a cumulative test and not focusing on what has been taught in class recently. Pay close attention to the material your professor has gone over, as well as any notes you may have taken during lecture time
- Not understanding the meaning of a word
- Forgetting to write down what they just read
- Ignoring the problems at hand and hoping one will solve itself
- Studying the wrong material
- Attempting to study too many topics at once and not focusing on one specific subject area or topic – Studying the wrong material
- Attempting to study too many topics at once and not focusing on one specific subject area or topic.
How to study for the HESI A2 Chemistry test?
- Read your HESI A2 chemistry book and our HESI Chemistry Formulas (or any other equivalent resource) and take notes
- Make sure to understand examples in the text by reviewing them, asking questions about difficult concepts, and looking up definitions of words you don’t know
- Watch YouTube videos that are related to chemistry or watch a lecture on the topic
- Take good notes in class so that they can be your study guide for tests
- Study with friends! Mimicking each other’s answers is an effective way to learn new material
Tips on how to succeed on the HESI A2 chemistry exam
In this chemistry study guide, we recommend you these useful tips to pass the exam:
- Always have a study plan and stick to it! For example, you might want to do one chapter each day for three days before your test. Make sure that there is time in between studying sessions where you rest so that you can absorb new information more effectively.
- Take the exams in a testing environment. You might not be used to taking tests in your regular classroom, but practicing on test day is important because it may feel more like you’re taking an actual exam
- Set up realistic expectations for what kind of grades you will get and make sure that you are reaching out to your professor if you have any questions
- Read the instructions carefully. Be sure that you are answering multiple-choice and true/false questions correctly because they count for more points and could be harder to figure out if you get them wrong
- Get plenty of sleep! This seems like a no brainer, but it’s easy to forget when you’re cramming the night before your test
- Don’t let yourself get too hungry or thirsty. You need to have a clear head in order to answer questions and you’ll be more likely to choke on difficult words if you’re thinking about food!
- Make sure that there is enough time for everything, but don’t go overboard. You don’t want to spend too much time on any one question and get stuck there.
- If possible, study with a group of friends or classmates. You’ll have more fun studying if you’re able to hang out and laugh while taking breaks!
- Get your thoughts down on paper before the test so that it’s easier for you to organize them. This will make it feel more like you’re doing a study guide and less stressful overall
- Don’t let yourself get too high or low based on how well you did the first time through an exam or quiz! You should take breaks to refresh your mind by playing games, reading articles, listening to music, etc.
- Review your notes after you’ve taken a break and make sure that there are no gaps in your knowledge!
- If you know the answer to a question but can’t remember it, try using an acronym or mnemonic device. You may need to look things up if these devices don’t work for you. Use whatever works for you! This will help the information stick in your memory
- If you’re taking a break from studying, try closing out all other windows and apps so that they don’t distract you. Also, turn off notifications on your phone or computer while doing this to avoid distractions
- Don’t get frustrated if what feels like an obvious answer to a question eludes you. You can always try another method of studying, do some research online, or ask your peers for help.
Study for the HESI chemistry test with our free HESI A2 Chemistry Practice Test
Hesi A2 Chemistry Practice Test Resource
Where to get Hesi A2 Test Resources and Materials? You should use our online HESI practice tests, HESI A2 study guide with detailed explanations, and brief evaluations to gauge your learning. By providing you with a solid math foundation, it can aid in your exam day preparation. Also, to help you gain a better understanding, hundreds of practice questions are broken down into distinct categories (exam subjects).
Now let’s practice!

PCCN vs CCRN: Which Certification Should I Take?
In this discussion, we will examine the fundamental distinctions between PCCN vs CCRN certifications, allowing you to make an informed and right decision about which certification is best for your nursing career progression.
June 20, 2023

Is PCCN Worth It? A Comprehensive 2025 Study Guide
In this article, we will provide all the enrollment criteria, how to apply, whether is PCCN worth it for you to obtain, and how to get a high mark.
June 20, 2023

PCCN Requirements - How to Become a Progressive Care Certified Nurse?
To become a progressive care nurse, you must first obtain the PCCN certification. This post will help you understand PCCN certification, PCCN requirements, and efficient approaches to obtaining this certification.
June 20, 2023
